Emission Spectra Diagram . in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. illustration of the hydrogen emission spectrum; Absorption spectra are lit with dark bands;. every atomic element has a unique absorption and emission spectrum. you need to be able to explain how an emission line is produced in an emission spectrum. Graph of hydrogen’s emission spectrum; You need to state that an. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is.
from www.vernier.com
the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Absorption spectra are lit with dark bands;. you need to be able to explain how an emission line is produced in an emission spectrum. You need to state that an. every atomic element has a unique absorption and emission spectrum. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. Graph of hydrogen’s emission spectrum; illustration of the hydrogen emission spectrum;
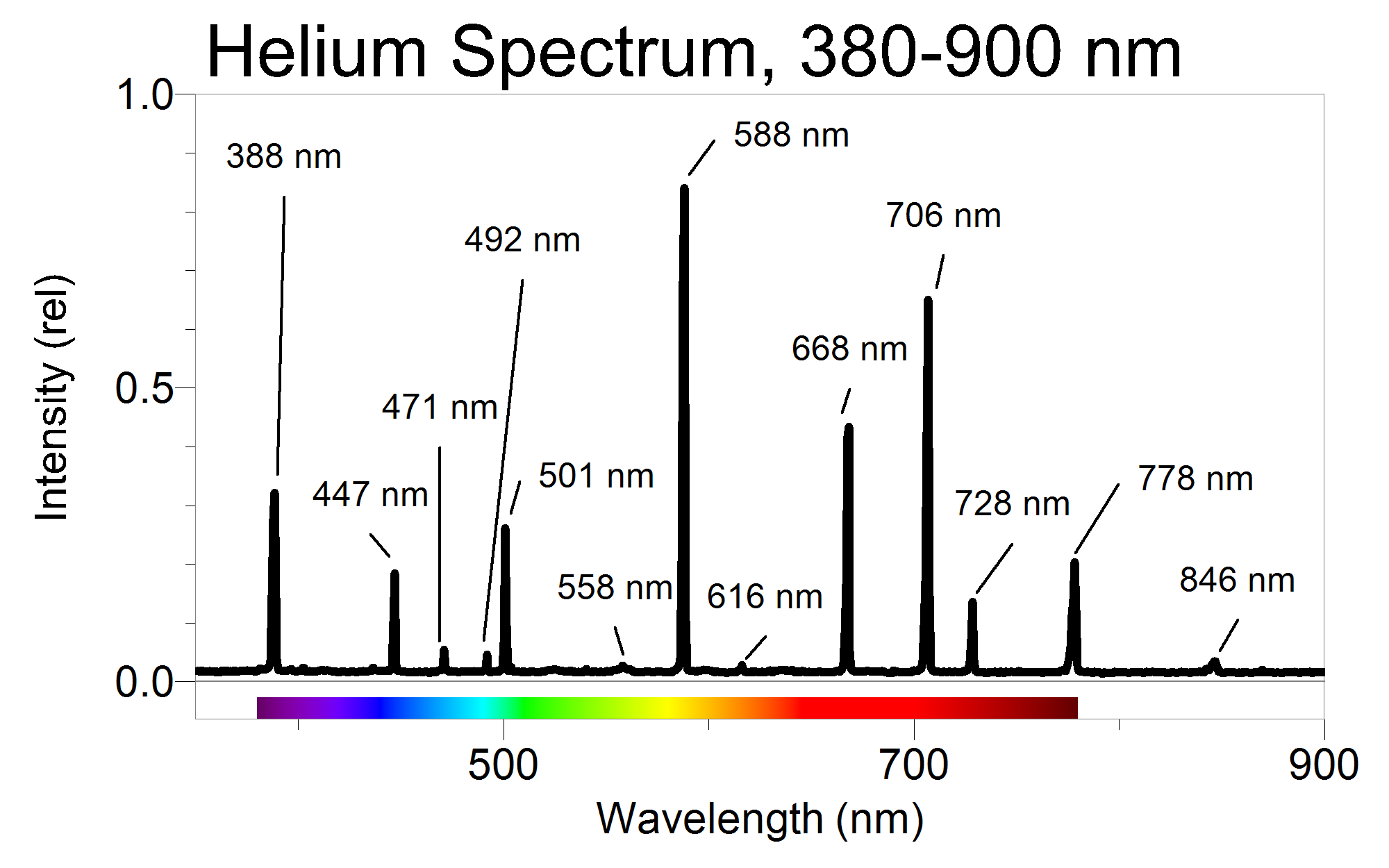
A Quantitative Investigation of the Helium Spectrum
Emission Spectra Diagram you need to be able to explain how an emission line is produced in an emission spectrum. You need to state that an. illustration of the hydrogen emission spectrum; Absorption spectra are lit with dark bands;. Graph of hydrogen’s emission spectrum; you need to be able to explain how an emission line is produced in an emission spectrum. every atomic element has a unique absorption and emission spectrum. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Spectra Diagram you need to be able to explain how an emission line is produced in an emission spectrum. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. You need to state that an. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the. Emission Spectra Diagram.
From www.pinterest.com
Emission Spectra and the Bohr Model Bohr model, Emissions, Model Emission Spectra Diagram the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of. Emission Spectra Diagram.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Diagram Graph of hydrogen’s emission spectrum; atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. Absorption spectra are lit with dark bands;. every atomic element has a unique absorption and emission spectrum. you need to be able to explain how an emission line is. Emission Spectra Diagram.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Emission Spectra Diagram You need to state that an. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. Absorption spectra are lit with dark bands;. Graph of hydrogen’s emission spectrum; atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. Web. Emission Spectra Diagram.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Diagram the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. illustration of the hydrogen emission spectrum; atomic emission spectra the electrons in an atom tend to be arranged in. Emission Spectra Diagram.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts Emission Spectra Diagram in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. Graph of hydrogen’s emission spectrum; the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. every atomic element has a unique absorption and emission spectrum. You need to state that. Emission Spectra Diagram.
From www.researchgate.net
Excitation and emission spectra’s of pyranine Download Scientific Diagram Emission Spectra Diagram atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. you need to be able to explain how an emission line is produced in an emission spectrum. Absorption spectra are lit with dark bands;. You need to state that an. every atomic element has. Emission Spectra Diagram.
From www.pinterest.com.mx
Emission Spectrum Vs. Absorption Spectrum Astronomy lessons, Spectrum Emission Spectra Diagram you need to be able to explain how an emission line is produced in an emission spectrum. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. every atomic element has a unique absorption and emission spectrum. Graph of hydrogen’s emission spectrum; Absorption spectra. Emission Spectra Diagram.
From www.chegg.com
Solved (7\) Problem 5 The diagrams below depict spectra Emission Spectra Diagram atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. illustration of the hydrogen emission spectrum; Graph of hydrogen’s emission spectrum; you need to be able to explain how an emission line is produced in an emission spectrum. You need to state that an.. Emission Spectra Diagram.
From lessonlibkindnesses.z21.web.core.windows.net
Star Spectrum Worksheet Emission Spectra Diagram the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. you need to be able to explain how an emission line is produced in an emission spectrum. Absorption spectra are lit with dark bands;. Graph of hydrogen’s emission spectrum; in this tutorial, you will learn about. Emission Spectra Diagram.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Diagram every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands;. Graph of hydrogen’s emission spectrum; illustration of the hydrogen emission spectrum; in this tutorial, you will learn about emission spectrum, absorption spectrum, and what is a spectrophotometer. you need to be able to explain how an emission line is. Emission Spectra Diagram.
From manuallistjackshaft.z22.web.core.windows.net
Electron Transition Diagram Emission Spectra Diagram you need to be able to explain how an emission line is produced in an emission spectrum. illustration of the hydrogen emission spectrum; the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. You need to state that an. every atomic element has a unique. Emission Spectra Diagram.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Diagram You need to state that an. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Absorption spectra are lit with dark bands;. every atomic element has a unique absorption and emission spectrum. illustration of the hydrogen emission spectrum; in this tutorial, you will learn. Emission Spectra Diagram.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Diagram Absorption spectra are lit with dark bands;. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. illustration of the hydrogen emission spectrum;. Emission Spectra Diagram.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Emission Spectra Diagram atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. every atomic element has a unique absorption and emission spectrum. You need to state that an. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when. Emission Spectra Diagram.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Diagram you need to be able to explain how an emission line is produced in an emission spectrum. every atomic element has a unique absorption and emission spectrum. illustration of the hydrogen emission spectrum; atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is.. Emission Spectra Diagram.
From protonstalk.com
Difference Between Emission and Absorption Spectra ProtonsTalk Emission Spectra Diagram You need to state that an. atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is. you need to be able to explain how an emission line is produced in an emission spectrum. the emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Diagram.
From www.doorsteptutor.com
CSIR (Council of Scientific & Industrial Research) Physical Sciences Emission Spectra Diagram You need to state that an. you need to be able to explain how an emission line is produced in an emission spectrum. Absorption spectra are lit with dark bands;. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. every atomic element has a unique. Emission Spectra Diagram.